Quality and reliability
Compliance with ICH GCP and successfully passed inspections
According to World Health Organization, Compliance with GCP provides public assurance that the rights, safety, and well-being of research subjects are protected and respected, consistent with the principles enunciated in the Declaration of Helsinki and other internationally recognized ethical guidelines and ensures the integrity of clinical research data.
GCT is fully compliant with ICH GCP – our Quality Assurance department oversees keeping GCT up to date and training GCT staff in accordance with the current regulations.
The evidence of above can be proved by:
- GCT has been inspected by U.S. Food and Drug Administration (FDA).
- In 2015, the State Expert Center of Ministry of Health of Ukraine conducted the audit at GCT – no findings were found, confirming GCT compliance with local laws, as well as ICH GCP standards.
- In 2016, Roszdravnadzor, subordinate to the Ministry of Health of the Russian Federation, carried out the inspection with no major findings were revealed.
Risk management covers a series of activities or processes that are carried out throughout the life cycle of a clinical trial in order to identify, assess, monitor, control, prevent, mitigate, inform and analyze any factor (or process) that endangers trial quality.

GCT was recently rated based on the business credit information by the world’s most credible and comprehensive source of commercial data. GCT scored 76 out of 80, which places the company at the top tier in our competitive industry.
Our partners can be certain that along with the highest quality of contract research services provided by GCT worldwide there is creditworthy and significant value behind our business relationships.
developed SOPs
trainings and staff
project-related trainings
Management
(offices, facilities,
departments, projects)
oversight
qualification
to the specific tasks
or duties


developed SOPs
trainings and staff
project-related trainings
Management
(offices, facilities,
departments, projects)
oversight
qualification
to the specific tasks
or duties
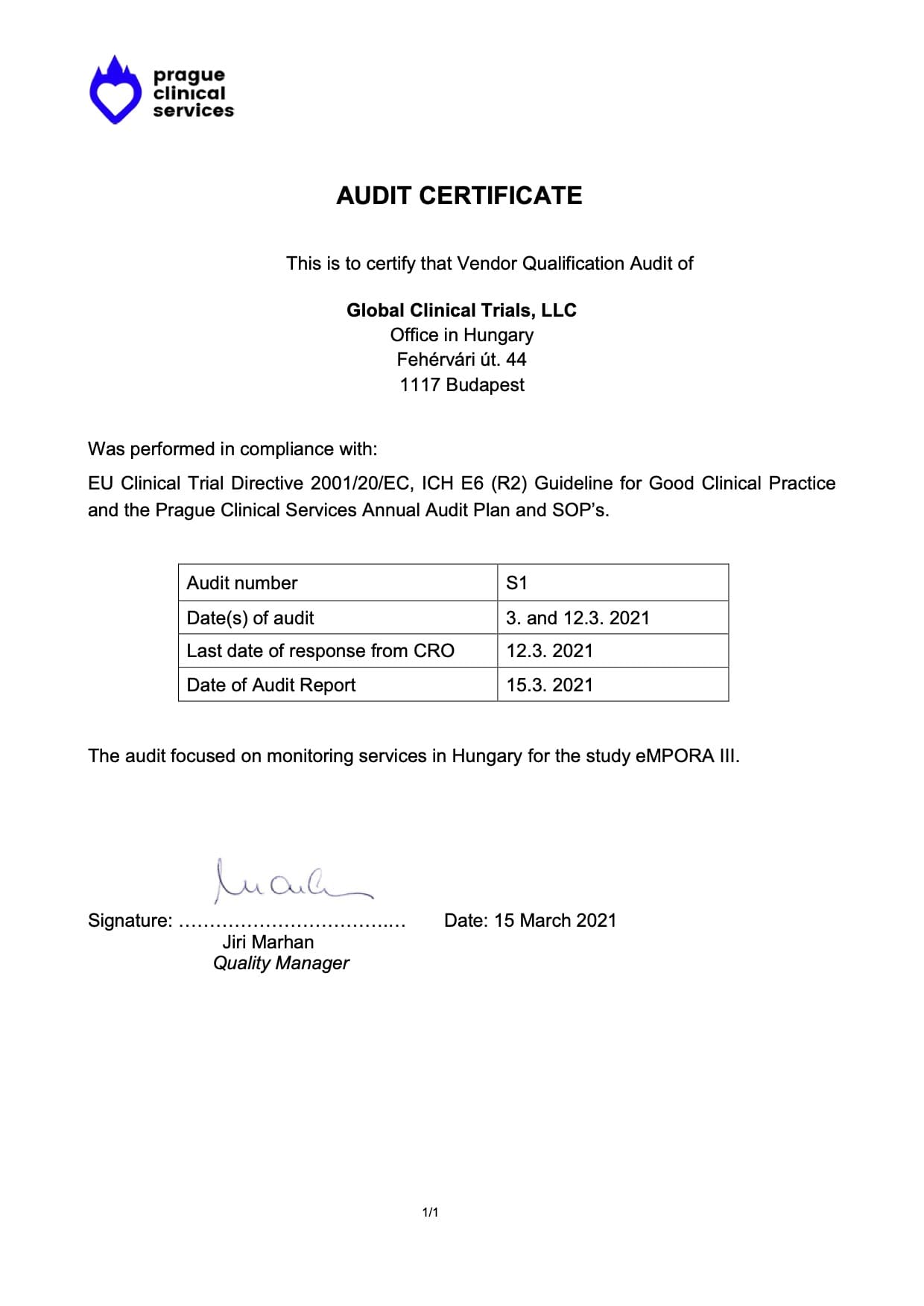
Audit Certificates




Let’s keep in touch!
Get the latest news about GCT and the projects we support in a monthly email. View the mailing archive