Regulatory
Hungary is located in Central Europe and has population of 9.77 million (World Bank, 2019). Its biggest city and a capital is Budapest, with a population of 1.75 million.
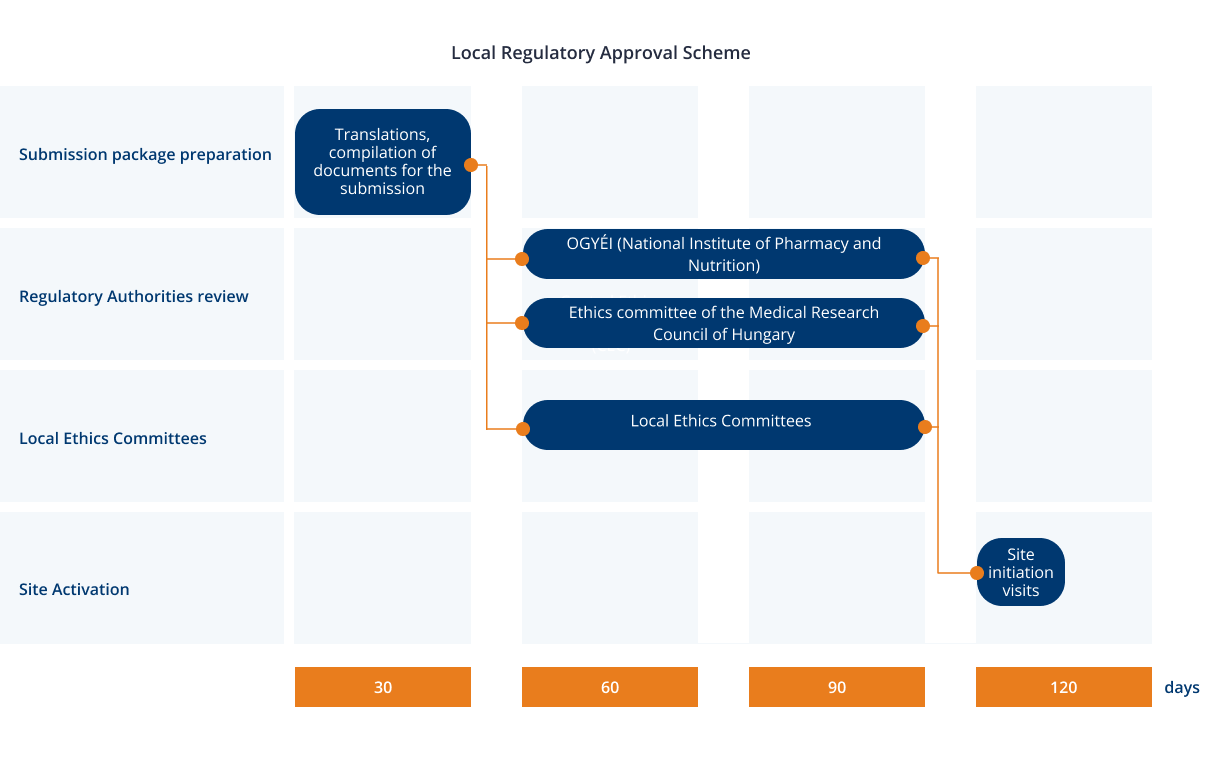
In Hungary, clinical trial legislation was harmonized with the European standards in 2004. The approval process is relatively quick and straightforward. It is performed via a single authority — the Hungarian National Institute of Pharmacy and Nutrition (OGYÉI). OGYÉI is responsible for various aspects of drug production, registration, and pharmacovigilance.
Official links
The Regulatory Authority (RA) typically completes the review of a study within 60-days. Central Ethics Committee (CEC) reviews the study in parallel and usually provides the approval sooner than the RA. Approval from the LECs (Local Ethics Committees) is not required to begin the study. LECs oversee the clinical trial at the assigned sites.