Regulatory
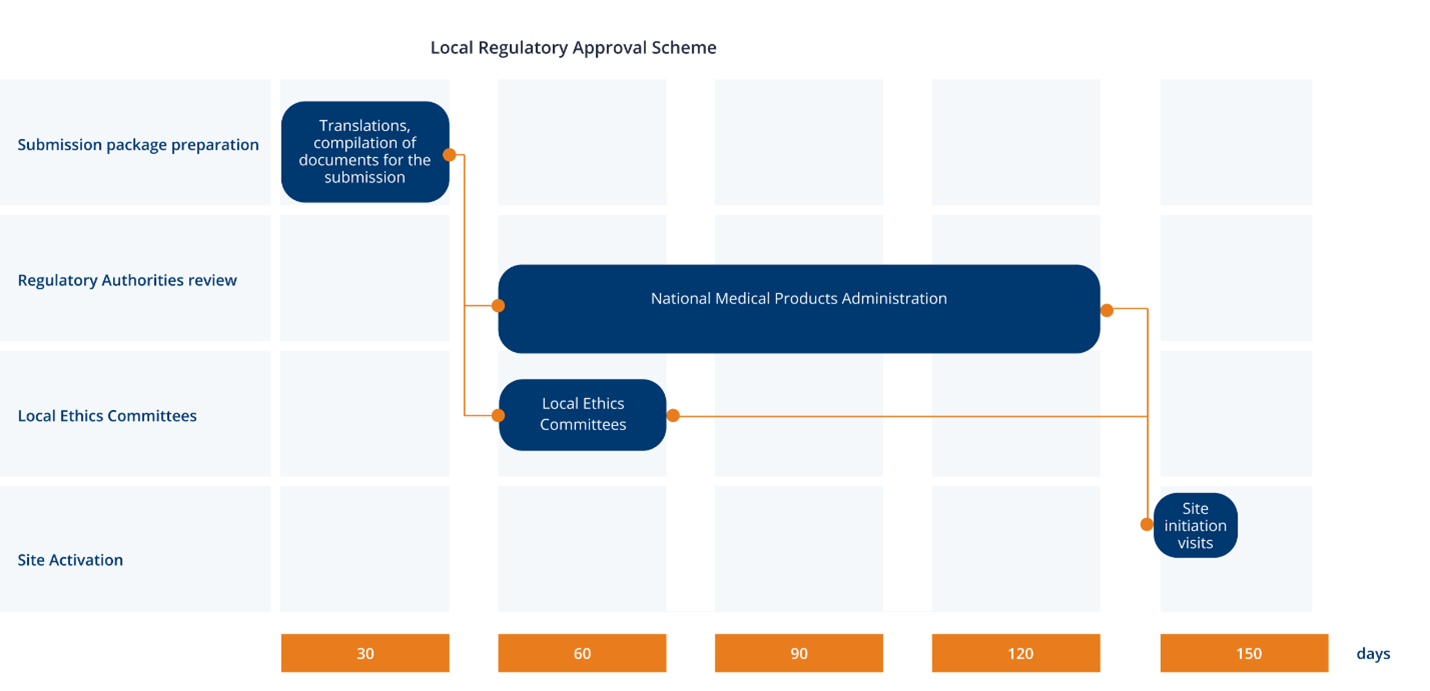
The Chinese agencies for regulating drugs and medical devices are The National Medical Products Administration (NMPA) and the Ministry of Science and Technology (MOST). Clinical trial applications can be submitted to NMPA for Phase I through Phase IV clinical trials, including bioequivalence trials, that are conducted in China. Clinical trial application will be considered approved after 60 working days if the applicant does not receive a rejection or an inquiry for clarification from the NMPA.
An ethics committee (EC) must approve a clinical trial application prior to a sponsor initiating a clinical trial. The EC review may be submitted in parallel to the NMPA’s review, but the study cannot be initiated until after review and approval by the EC. The EC must give written notice of its decision to the applicant within 10 working days after the review.
Official links
Link: https://clinregs.niaid.nih.gov/country/china#safety_reporting