GCT has been awarded a new clinical trial in Ukraine and Russia.
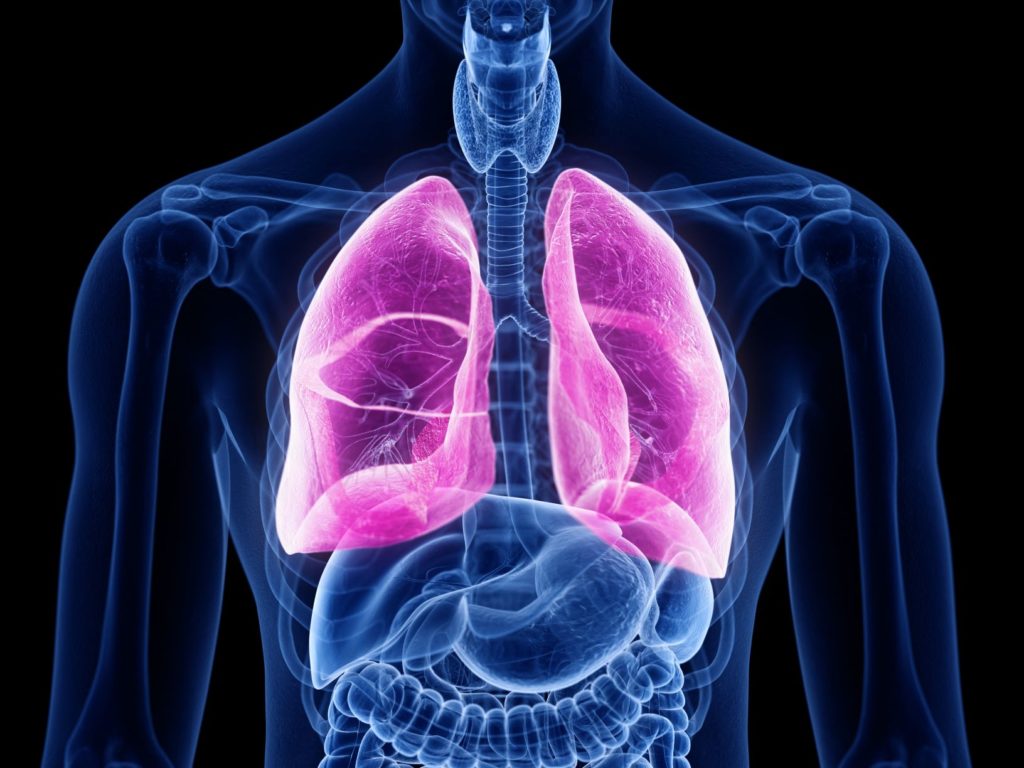
A Phase II multinational study in subjects with Idiopathic Pulmonary Fibrosis (IPF) is designed to investigate the safety, efficacy, and pharmacokinetics of the investigational product. IPF is a disease that is characterized by scarring of the lungs. As condition develops, it becomes harder and harder to breathe. There’s no cure for this disease, however medications can help manage the symptoms.
We are providing full-service clinical support in both countries, including feasibility analysis and site identification, regulatory support, monitoring, site management and financial management. The trial is currently in the start-up stage in Ukraine and Russia. Regulatory submission preparations and pre-study visits to the selected sites are in progress.
“Idiopathic Pulmonary Fibrosis is a serious and devastating disease, often with poor prognosis. We look forward to conducting this important trial aimed at improvement of patients’ quality of life. It enhances our extensive experience in rare diseases and, ultimately, will provide patients with more treatment options,” – Dr. Jeffrey Apter, GCT President.