Regulatory
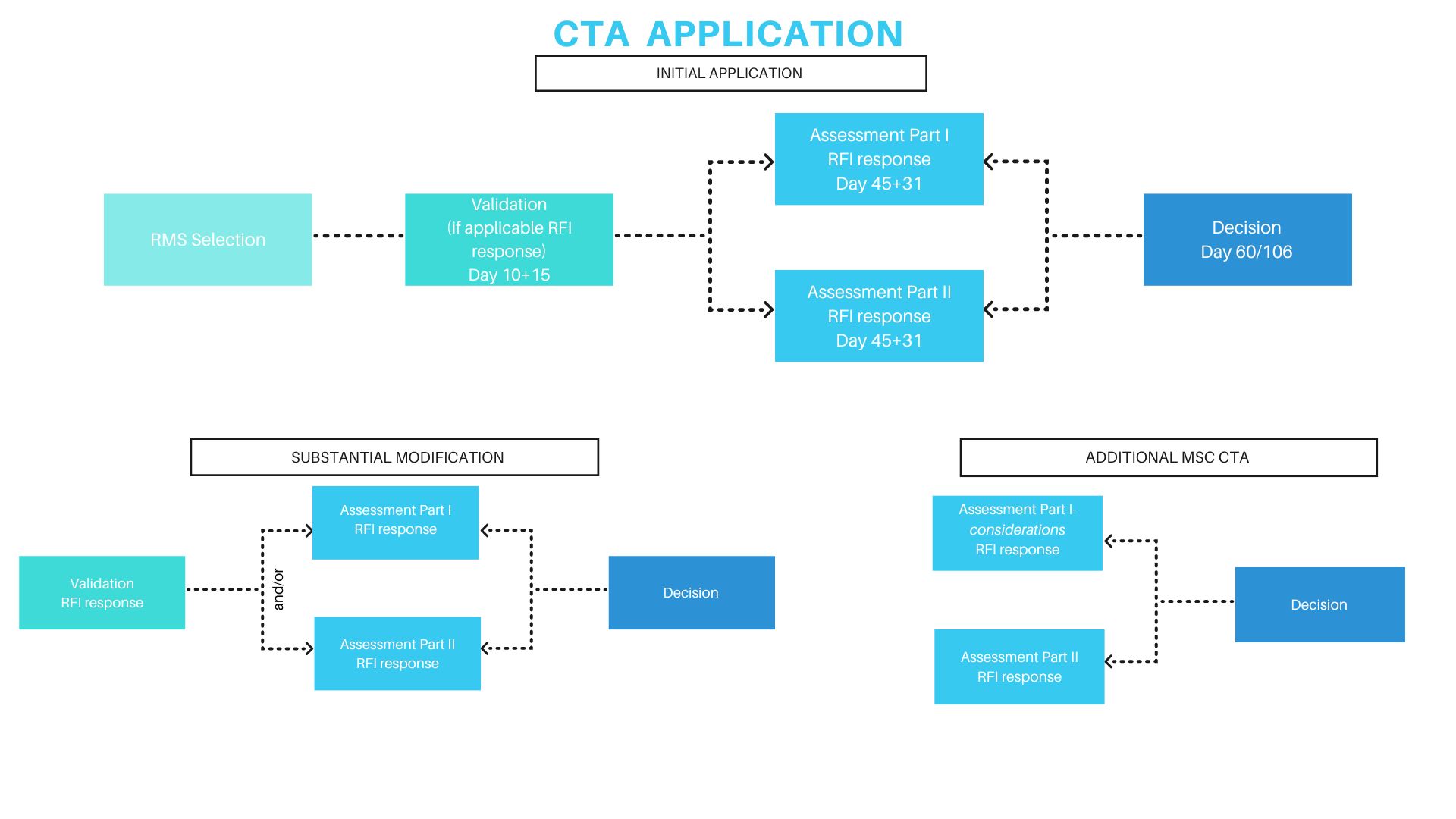
Under Regulation (EU) No 536/2014, clinical trials in the EU are authorized through the Clinical Trials Information System (CTIS). A sponsor submits one harmonized application via the EU-portal for all intended Member States. The dossier is split into:
- Part I (scientific and technical documentation, common to all CMS)
- Part II (national documents, including ethics requirements)
One Reporting Member State (RMS) coordinates the scientific review, which must be completed within 45 days, with parallel national (Part II) reviews. Requests for information may pause the clock. Each Member State must then issue a decision within 5 days, leading to an overall maximum of 60 days (extensions possible for complex therapies).
The CTR harmonizes approvals across the EU, integrates ethics committees into the process, and ensures transparency through the EU trial database. Since 31 January 2023, all new trials in the EU must follow this system.