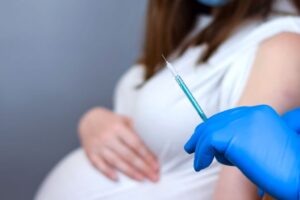
GCT has been awarded a multicenter flu vaccine clinical trial in Russia. It will be carried out with participation of approximately 500 pregnant women at screening, and with a follow-up extension to babies. GCT possesses previous experience in conducting multinational studies in pregnant women with participation of 1000+ subject.
The sponsor, one of the largest research institutes in the country set strict requirements for CRO qualification process and challenging study parameters. Hence, GCT is proud to be the only local CRO eligible for the study conduct after the public tender procedure.
“GCT possesses solid resources and proven capabilities in Russia. We are confident to support this important study in cooperation with the client at the highest international standard of quality,” – said Dr. Eugene Selivra, GCT CEO.
The regulatory approval has already been obtained, and the study will be managed by GCT full-service onward. GCT logistics department will also support the supply and import of the comparator vaccine from oversees, utilizing the warehouse facilities owned in Russia. In order to minimize the number of onsite subject visits during COVID-19, the team considers using ePRO solutions for data collection at follow up visits.