We are delighted to announce that both EC and RA approvals to conduct clinical trial in Benign Prostatic Hyperplasia patients were received by GCT team earlier this week. We are preparing for the site initiation in order to start screening and enrollment procedures in November.
Benign Prostatic Hyperplasia is the non-cancerous enlargement of the prostate, affecting about 50% of men above 60 years of age and more than 70% of men ages 70 and above. Please contact us at bd@gctrials.com to navigate through the clinical, regulatory and logistical aspects of conducting device and pharmaceutical trials in Slovakia and other EU countries.
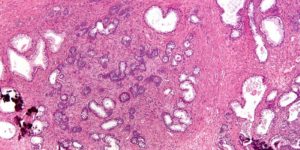
Illustration: Micrograph of nodular hyperplasia of the prostate, also known as benign prostatic hyperplasia (BPH) and benign prostatic hypertrophy. Author.