 GCT has worked closely with the Sponsor on reaching this important milestone and over the past months has secured Regulatory and EC approvals, conducted site initiation visits and managed shipments of required supplies to the sites. We have successfully progressed to the enrollment start on schedule in the two participating countries — Hungary and Poland.
GCT has worked closely with the Sponsor on reaching this important milestone and over the past months has secured Regulatory and EC approvals, conducted site initiation visits and managed shipments of required supplies to the sites. We have successfully progressed to the enrollment start on schedule in the two participating countries — Hungary and Poland.
Recruitment was preceded by an Investigator meeting organized locally by the GCT team, bringing together Sponsor, key site personnel, Central Laboratory representatives and GCT monitors for joint training on the protocol and specific study procedures, as well as for other important discussions and review of such crucial aspects as handling of the IP and CTMs.
This important program will not only provide access to alternative, more convenient treatment options to the patients in need but will also allow GCT and Sponsor to strengthen relations with the KOLs and local European ophthalmology community
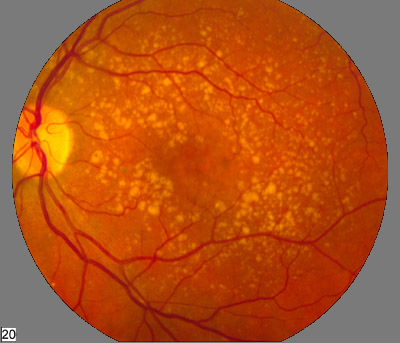
Pictured: A fundus photo showing intermediate age-related macular degeneration.
Credit: National Eye Institute, National Institutes of Health, USA